REFLECTION TOPIC
Process Validation in the Bio/Pharmaceutical Industry
In 2011 the FDA issued a Guidance for Industry on process validation. The guidance formally defined stages during the lifecyle of a commercial process design. These are as follows:
- Stage 1: Process Design. Analytical and process development activities, prior platform knowledge, published literature and other sources of knowledge are generated and gathered. These may be inputs to the design of a commercial process. Based on the FDA definition, “Process design is the activity of defining the commercial manufacturing process that will be refected in planned master production and control records. The goal of this stage is to design a process suitable for routine commercial manufacturing that can consistently deliver a product that meets its quality attributes”.
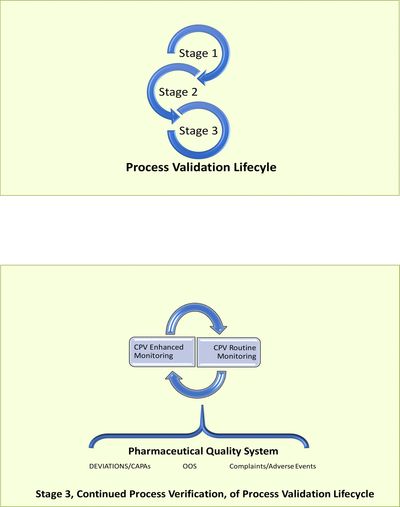
- Stage 2: Process Validation. This stage is further subdivided into Stage 2a: facility and equipment qualification and Stage 2b: Process Perforamance Qualification. Stage 2b or "PPQ combines the actual facility, utilities equipment (each now qualified) and the trained personnel with the commercial manufacturing process, control procedures, and components to product commercial batches. A successful PPQ will CONFIRM the process design and demonstrate that the commercial manufacturing process performs as expected.”
- Stage 3: Continued Process Verification entails continued monitoring of process performance throughout the remaining lifecyle of the commercial process. The use of statistics is encouraged as a means to quantitatively measure and identify potential points/sources of departures (special causes of variation) from the typical performance of the process (common cause variations). In addittion, continued monitoring provides an opportunity for improvement. “The goal of the third validation stage is continual assurance that the process remains in a state of control (the validated state) during commercial manufacture. A system or systems for detecting unplanned departures from the process as designed is essential to accomplish this goal. Adherence to the cGMP requirements, specifically, the collection and evaluation of information and data about the performance of the process, will allow detection of undesired process variability. Evaluating the performance of the process identifies problems and determines whether action must be taken to correct, anticipate and prevent problems so that the process remain in control.” ...... “An ongoing program to collect and analyze product and process data that relate to product quality must be established. The data collected should include relevant process trends and quality of incoming materials or component, in-process material, and finished products. The data should be statistically trended and reviewed by trained personnel. The information collected should verify that the quality attributes are being appropriately controlled throughout the process.”
Transition to Continued Process Validation (CPV)
During stages 1 and 2b statistics is frequently implemented in the design of experiments. However, in particular for the development of a biological commercial process for the manufacturing of API/DS, further implementation of statistical tools is restricted due to the availability of material, sample sizes as well as, scale and facility considerations. At the start of stage 3, the FDA agency recommends a “continued monitoring and sampling of process parameters and quality attributes at the level established during the process qualification stage until sufficient data are available to generate significant variability estimates” Proper application of statistics requires unimodality of distribution curves, as well as, randomness and independence of data. Care must be taken to ensure that during the application of process monitoring tools when transitioning between Stage 2 to Stage 3 the above prerequisites are met. It is critical to have proper sample sizes, normality and a stable process (common cause variation only) prior to calculating process capabilities. This can be of particular importance in the development of biologics that rely significantly in outsourced services and use multiple contract manufacturing organizations (CMOs) to meet supply demands.
Find Out More
Other References:
Evolution of Biopharmaceutical Control Strategy through Continued Process Verification,
BioProcess International. Vol 15(1) January 2017
Veraz Biosolutions
P.O. Box 98434
Raleigh, NC 27624